
- Science Toys
- Magnetism
- Electromagnetism
- Electrochemistry
- Radio
- Thermodynamics
- Aerodynamics
- Light and optics
- Simple laser communicator
- Make your own 3D pictures
- Making permanent rainbows.
- A solar powered marshmallow roaster
- Make a spectroscope from a CD.
- The impossible kaleidoscope
- Make a solar hotdog cooker
- Exploring invisible light
- A high resolution spectrograph.
- Time-lapse photography.
- High speed photography.
- Stacking photos for high depth of field.
- Biology
- Mathematics
- Computers and Electronics
A metal alloy that is liquid at room temperature.
Suppose you had a metal alloy that had the advantages of liquid mercury, but without the toxic effects? You could make your own barometers and thermometers, and not worry about calling in a hazardous materials team to clean up after any accidents. You could simply wipe up the mess with a paper towel. You wouldn't have to worry about breathing in toxic mercury fumes, but you could still make neat little electric motors that dip into liquid metal to make their electrical connections. Suppose further, that the metal would stick to glass, so you could paint it on glass to make your own mirrors. Or that it would stick to paper so you could draw your own electric circuits in it?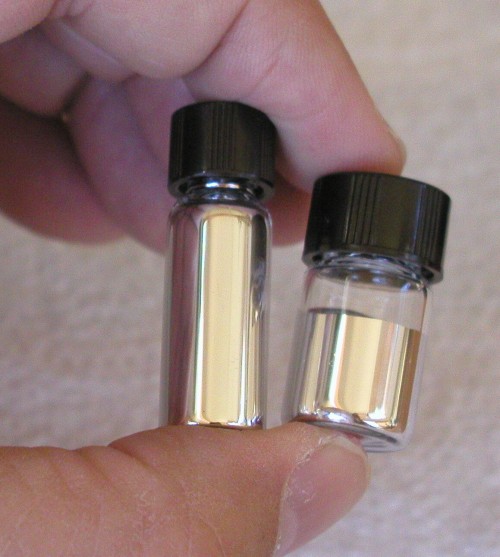
Click on photo for a larger picture In the photo above, I am holding two small vials of liquid metal. The vial on the right contains gallium, an element that melts at 29.76° Celsius (85.57° Fahrenheit). The vial on the left is an alloy that contains gallium, indium, and tin, and melts at -20° Celsius (-4° Fahrenheit). (Both are available in our catalog.) The gallium is liquid because I had the bottle in my shirt pocket, next to my warm body. At normal comfortable room temperatures it is a solid. Because gallium expands when it solidifies (unlike most metals), the vials are only filled half way. To get the solid metal out of the vial, simply warm it up in a cup of hot water until it melts.
Fun things to do with liquid metal
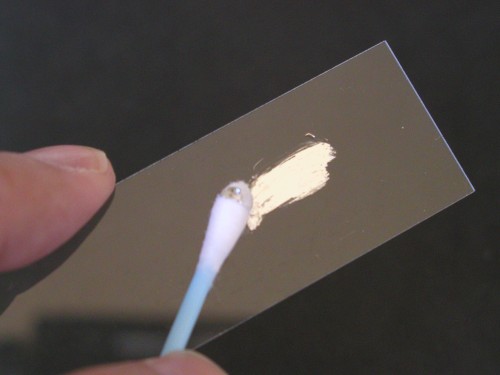
One fun thing you can do right away with the liquid metal alloy is make your own mirrors. All it takes is a piece of glass and a cotton swab.
Click on photo for a larger picture Dip the cotton swab in the vial, and twirl it around to coat it with the liquid metal alloy.
Click on photo for a larger picture Now rub the coated swab on the glass (in the phot we are using a glass microscope slide). The metal sticks to the glass, and makes an opaque reflective coating.

Click on photo for a larger picture In the photo above, I am holding the new mirror so that it reflects the view of the trees outside my window. The camera is focused on the window, so the trees and my hand are out of focus. Being able to make your own mirrors is an advantage when the mirror you need can't be bought anywhere. For example, I needed a small lightweight mirror to glue to a speaker, so I could bounce a laser beam off of the speaker and have the music wiggle the mirror, making a pattern on the wall.
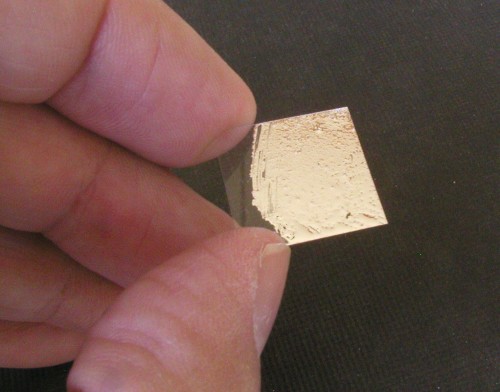
Click on photo for a larger picture I used the liquid metal to coat a thin glass cover slip for a microscope slide.
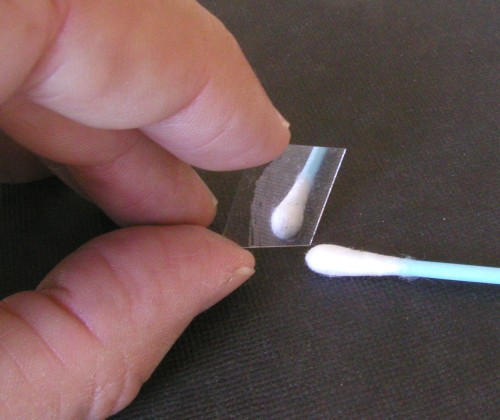
Click on photo for a larger picture The resulting mirror was very lightweight, and yet stiff, so it would remain flat while being bounced around by the speaker.
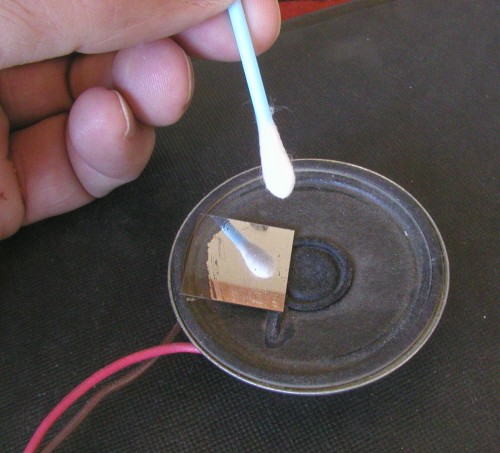
Click on photo for a larger picture When it is glued onto the speaker and the music turned on, the laser makes a light show on the wall. Using two speakers, and bouncing the light off of one and then off of the other, gives you two dimensions, and you can make a computer sound file that uses both stereo channels to draw pictures on the wall.
More fun things
There are lots of things you can do with liquid metal:- Make thermometers
- Make barometers
- Make tilt meter seismographs
- Make non-conductive objects conductive
- Make electrodes that conform to varying surfaces
- Experiment with magnetohydrodynamics
- Wiggle it with high frequency electricity
- Use it to conduct high energy sound
- Replace mercury in spinning telescope mirrors
How does it do that?
Gallium is an element (atomic number 31, right below aluminum and just to the right of zinc in the periodic table of the elements). It starts out with a very low melting point already, but we can add some other elements to get an even lower melting point. Right below gallium in the periodic table is indium (element 49). Just to the right of indium is tin (element 50). When these elements are combined, their atoms bind together into a compound. The molecules of that compound do not bind to one another as much as the atoms of the original metals bound to each other. This lowers the melting point. There are many ways to combine the three metals:| Compound | Percentages | Grams Ga | Grams In | Grams Sn |
| Ga14In3Sn2 | 62.65% Ga, 22.11% In, 15.24% Sn | 97.6122 | 34.4454 | 23.742 |
| Ga17In4Sn2 | 62.98% Ga, 24.40% In, 12.62% Sn | 118.529 | 45.9272 | 23.742 |
| Ga22In5Sn3 | 62.25% Ga, 23.30% In, 14.45% Sn | 153.391 | 57.409 | 35.613 |
| Ga25In5Sn4 | 62.43% Ga, 20.56% In, 17.01% Sn | 174.308 | 57.409 | 47.484 |
| Ga25In6Sn3 | 62.52% Ga, 24.71% In, 12.77% Sn | 174.308 | 68.8908 | 35.613 |

Click on photo for a larger picture The alloy eventually combines with all of the aluminum, dissolving a hole in it.

Click on photo for a larger picture If a drop of water is added to the resulting bead of liquid metal, the water combines vigorously with the aluminum, making a hot solution of caustic aluminum hydroxide. What is left is the original drop of gallium, with a tiny amount of aluminum dissolved in it. (Don't put that drop back in the bottle, it will contaminate the rest of the gallium). This experiment can be done with either the gallium, or the gallium-indium-tin alloy. Next: Aerodynamics -- The Bernouli Ball Order Gallium and Liquid Metal Alloy here. Del.icio.us
- Science Toys
- Magnetism
- Electromagnetism
- Electrochemistry
- Radio
- Thermodynamics
- Aerodynamics
- Light and optics
- Simple laser communicator
- Make your own 3D pictures
- Making permanent rainbows.
- A solar powered marshmallow roaster
- Make a spectroscope from a CD.
- The impossible kaleidoscope
- Make a solar hotdog cooker
- Exploring invisible light
- A high resolution spectrograph.
- Time-lapse photography.
- High speed photography.
- Stacking photos for high depth of field.
- Biology
- Mathematics
- Computers and Electronics
Some of my other web sites:

Send mail to Simon Quellen Field via sfield@scitoys.com