Ingredients --
Ethylene diamine tetra acetic acid
Chemical Formula:
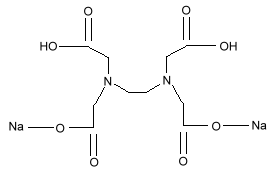
Sodium EDTA
Synonyms
Sodium EDTA,Calcium EDTA
Description
Uses
Sodium or calcium EDTA binds to metals, such as nickel, copper, and iron, making them unavailable to react with other ingredients in a product, or with compunds in the human body. Compounds that act in this way are called sequestering agents, or chelating agents (after the word for the claw of a crab -- chelae). EDTA is used to treat lead and mercury poisoning, as it can lock up those metals so they can do no harm in the body. EDTA also sequesters calcium and magnesium from hard water, preventing them from forming insoluble soap films (scum) with soaps and detergents. Chelators are sometimes used to sequester metal ions that interfere with dyes and perfumes. Compounds with similar functions are sodium carbonate, pentasodium pentetate, sodium citrate, phosphoric acid, tetrasodium etidronate, and tetrasodium pyrophosphate.edta: InChI=1/C10H16N2O8/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20)/f/h13,15,17,19H
sodium carbonate: InChI=1/CH2O3.Na/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-2/fCO3.Na/q-2;m
sodium citrate: InChI=1/C6H8O7.2Na/c7-3(8)1-6(13,5(11)12)2-4(9)10;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);;/q;2*+1/p-2/fC6H6O7.2Na/h11H;;/q-2;2m
tetrasodium edta: InChI=1/C10H16N2O8.4Na/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20;;;;/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20);;;;/q;4*+1/p-4/fC10H12N2O8.4Na/q-4;4m
phosphoric acid: InChI=1/H3O4P/c1-5(2,3)4/h(H3,1,2,3,4)/f/h1-3H
pentasodium pentetate: InChI=1/C14H23N3O10.5Na/c18-10(19)5-15(1-3-16(6-11(20)21)7-12(22)23)2-4-17(8-13(24)25)9-14(26)27;;;;;/h1-9H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25)(H,26,27);;;;;/q;5*+1/p-5/fC14H18N3O10.5Na/q-5;5m
By Simon Quellen Field