Ingredients --
Tetrasodium pyrophosphate
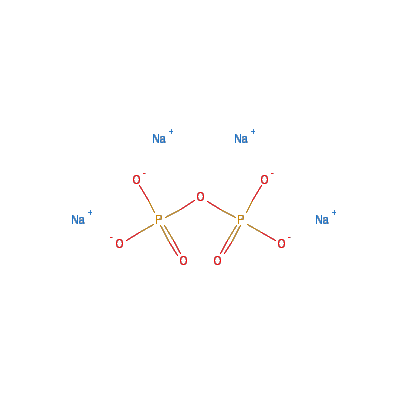
Chemical Formula: Na4O7P2
Synonyms
Sodium pyrophosphate,TSPP,
tetrasodium diphosphate
Description
Colorless transparent crystals or white powder.Uses
Tetrasodium pyrophosphate is used as a pH buffer (a substance which maintains a particular acidity level), and as a dough conditioner in soy-based "meat alternatives". It promotes binding of proteins to water, binding the soy particles together, and is used for the same purpose in chicken nuggets and imitation crab and lobster products. It is an emulsifier, and a source of phosphorus as a nutrient. It is used in toothpastes, as a buffer, an emulsifier, and a detergent aid. It is the "tartar control" agent. It removes calcium and magnesium from the saliva, so they can't deposit on the teeth. It is a thickening agent in instant puddings. It is a water softener in detergents, and an emulsifier to suspend oils and prevent them from redepositing on clothing in the wash. As a water softener, it combines with magnesium to sequester it from the detergent, without precipitating it onto the clothing. As a detergent additive, it can also "reactivate" detergents or soaps that have combined with calcium to make an insoluble scum. The TSPP sequesters the calcium, replacing it with sodium, which reactivates the detergent or soap, and yet keeps the calcium from precipitating out of solution. Because phosphates cause "eutrophication" of water (algae grows because of the fertilizing power of phosphates), it is seldom used as a detergent additive, except in toothpastes.Tetrasodium pyrophosphate: InChI=1/4Na.H4O7P2/c;;;;1-8(2,3)7-9(4,5)6/h;;;;(H2,1,2,3)(H2,4,5,6)/q4*+1;/p-4/f4Na.O7P2/q4m;-4
By Simon Quellen Field