Delivert
 PubChem Notes:
PubChem Notes:
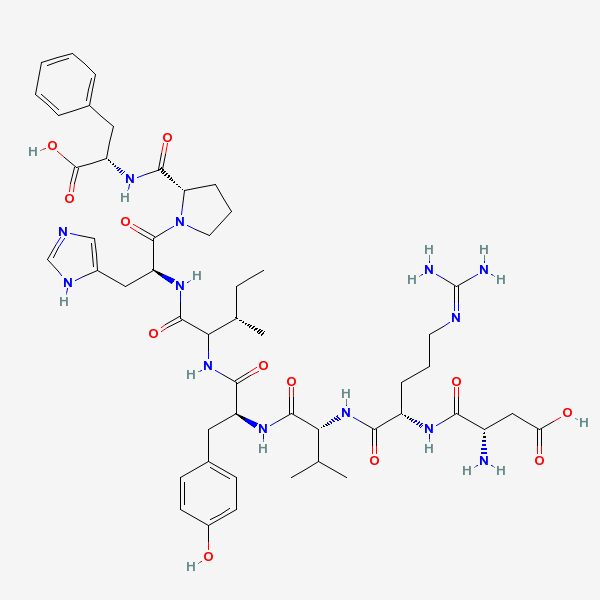
Angiotensin II The active form of angiotensin. An octapeptide found in blood, it is synthesized from ANGIOTENSIN I and quickly destroyed. Angiotensin II causes profound vasoconstriction with a resulting increase in blood pressure. It differs among species by the amino acid in position 5. The human form has ISOLEUCINE in this position. The clinically and experimentally used bovine form has VALINE in position 5. Medically useful antagonism is obtained with ACE INHIBITORS or with ANGIOTENSIN II TYPE 1 RECEPTOR BLOCKERS.
Molecular Formula:
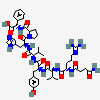
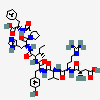
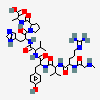
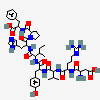
C50H71N13O12
InChI: InChI=1/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40+,41?/m0/s1/f/h56-62,65,74H,52-53H2
InChIKey: InChIKey=CZGUSIXMZVURDU-UKCCZYCCDJ
SMILES: CCC(C)C(C(=O)NC(CC1=CN=CN1)C(=O)N2CCCC2C(=O)NC(CC3=CC=CC=C3)C(=O)O)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(C(C)C)NC(=O)C(CCCN=C(N)N)NC(=O)C(CC(=O)O)N
Names:
Angiotensin II (human type) (JAN)
Angiotensin II (human type)
ANGIOTENSIN II
Delivert (TN)
Delivert
D02014
(3S)-3-amino-3-[[(1S)-1-[[(1R)-1-[[(1S)-1-[[(2S)-1-[[(2S)-1-[(2S)-2-[[(1S)-1-carboxy-2-phenyl-ethyl]carbamoyl]pyrrolidin-1-yl]-3-(3H-imidazol-4-yl)-1-oxo-propan-2-yl]carbamoyl]-2-methyl-butyl]carbamoyl]-2-(4-hydroxyphenyl)ethyl]carbamoyl]-2-methyl-propyl]carbamoyl]-4-(diaminomethylideneamino)butyl]carbamoyl]propanoic acid
4474-91-3
Registries:
PubChem CID 656651
PubChem ID 7849076
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|