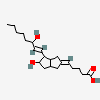
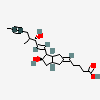
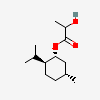ILOPROST
 PubChem Notes:
PubChem Notes:
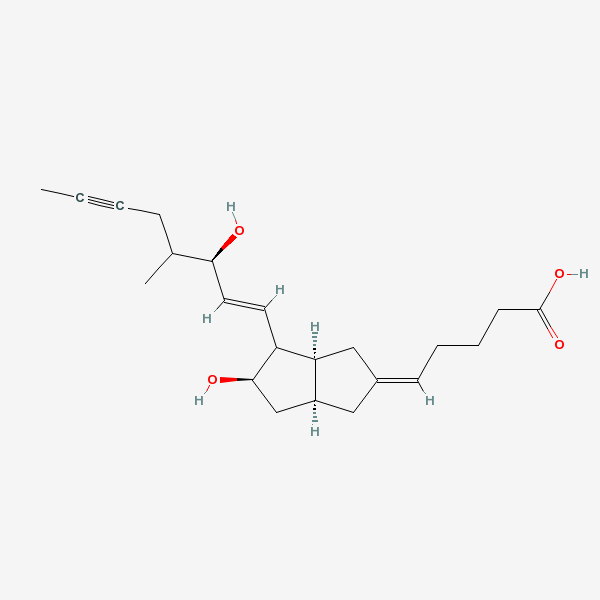
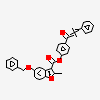
Iloprost An eicosanoid, derived from the cyclooxygenase pathway of arachidonic acid metabolism. It is a stable and synthetic analog of EPOPROSTENOL, but with a longer half-life than the parent compound. Its actions are similar to prostacyclin. Iloprost produces vasodilation and inhibits platelet aggregation.
Molecular Formula:
C22H32O4
InChI: InChI=1/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10u,16-8-/t15u,17-,18u,19-,20-,21+/m0/s1/f/h25H
InChIKey: InChIKey=HIFJCPQKFCZDDL-WVLWLIHTDG
SMILES: CC#CCC(C)C(C=CC1C(CC2C1CC(=CCCCC(=O)O)C2)O)O
Names:
ILOPROST
(5Z)-5-[(3aS,5R,6aR)-5-hydroxy-4-[(E,3R)-3-hydroxy-4-methyl-oct-1-en-6-ynyl]-3,3a,4,5,6,6a-hexahydro-1H-pentalen-2-ylidene]pentanoic acid
73873-87-7
Registries:
PubChem CID 6435293
PubChem ID 190653
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|