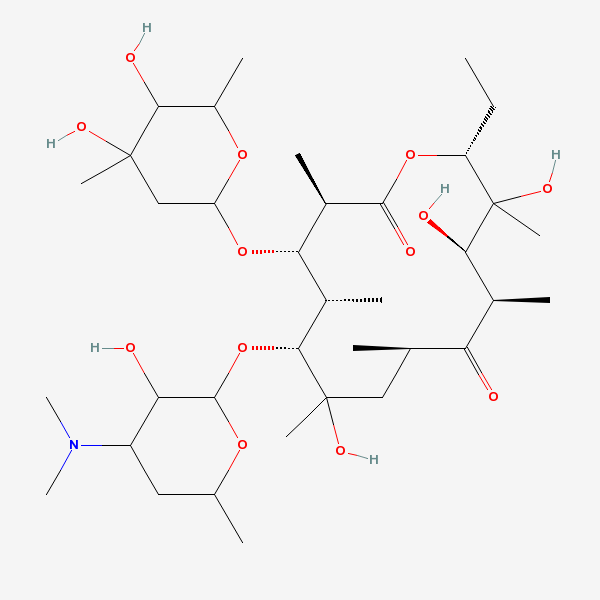
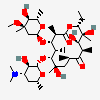
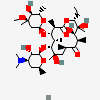
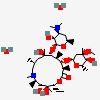
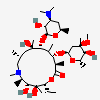
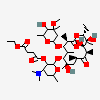
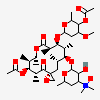
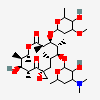
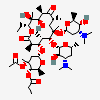
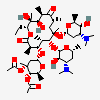
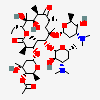
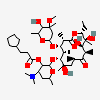
Erythromycin C
 PubChem Notes:
PubChem Notes:
Erythromycin A bacteriostatic antibiotic macrolide produced by Streptomyces erythreus. Erythromycin A is considered its major active component. In sensitive organisms, it inhibits protein synthesis by binding to 50S ribosomal subunits. This binding process inhibits peptidyl transferase activity and interferes with translocation of amino acids during translation and assembly of proteins.
Molecular Formula:
C36H65NO13
InChI: InChI=1/C36H65NO13/c1-13-24-36(10,45)29(40)19(4)26(38)17(2)15-35(9,44)31(50-33-27(39)23(37(11)12)14-18(3)46-33)20(5)28(21(6)32(42)48-24)49-25-16-34(8,43)30(41)22(7)47-25/h17-25,27-31,33,39-41,43-45H,13-16H2,1-12H3/t17-,18u,19+,20+,21-,22u,23?,24-,25?,27?,28+,29-,30?,31-,33?,34?,35?,36?/m1/s1
InChIKey: InChIKey=MWFRKHPRXPSWNT-UPSHANLCBB
SMILES: CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)O)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O
Names:
Erythromycin C
(3R,4S,5R,6R,9R,11R,12R,14R)-4-(4,5-dihydroxy-4,6-dimethyl-oxan-2-yl)oxy-6-(4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl)oxy-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione
Registries:
PubChem CID 6426643
PubChem ID 10486427
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|






















