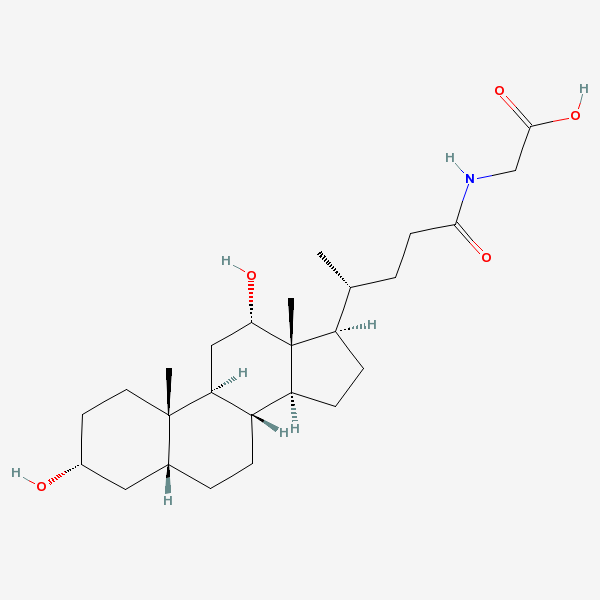
glycodesoxycholic acid
 PubChem Notes:
PubChem Notes:
Glycodeoxycholic Acid A bile salt formed in the liver by conjugation of deoxycholate with glycine, usually as the sodium salt. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is used as a cholagogue and choleretic.
Molecular Formula:
C26H43NO5
InChI: InChI=1/C26H43NO5/c1-15(4-9-23(30)27-14-24(31)32)19-7-8-20-18-6-5-16-12-17(28)10-11-25(16,2)21(18)13-22(29)26(19,20)3/h15-22,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16-,17-,18+,19-,20+,21+,22+,25+,26-/m1/s1/f/h27,31H
InChIKey: InChIKey=WVULKSPCQVQLCU-UZSHMJPJDW
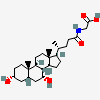
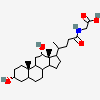
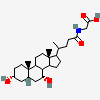
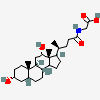
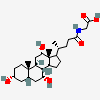
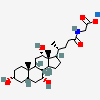
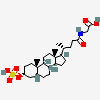
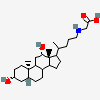
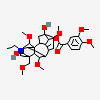
SMILES: [H][C@]12CC[C@@]3([H])[C@]4([H])CC[C@]([H])([C@H](C)CCC(=O)NCC(O)=O)[C@@]4(C)[C@@H](O)C[C@]3([H])[C@@]1(C)CC[C@@H](O)C2
CAS number 360-65-6
Names:
CHEBI:27471
Deoxycholic acid glycine conjugate
deoxycholic acid glycine conjugate
Deoxycholylglycine
deoxycholylglycine
Glycodeoxycholate
glycodeoxycholic acid
Glycodesoxycholic acid
glycodesoxycholic acid
N-(3alpha,12alpha-dihydroxy-5beta-cholan-24-oyl)glycine
2-[[(4R)-4-[(3R,5R,8S,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]acetic acid
Registries:
PubChem CID 3035026
Beilstein =2954947
CAS 360-65-6 (from NIST)
ChEBI 27471
Kegg C05464
PubChem ID 17425128
PubChem ID 7824
|
|
|
|
|
|
|
|
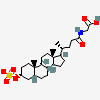
|
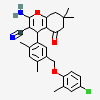
|
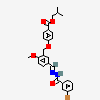
|
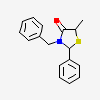
|
|
|
|
|
|
|
|
|
|