Ingredients --
Sodium stearate
Chemical Formula: CH3(CH2)16COONa)
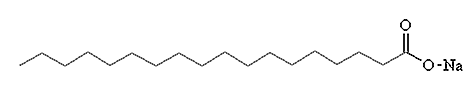
Synonyms
SoapDescription
Sodium stearate is one of the main compounds in common soap. To make soap, you start with beef fat. If you treat beef fat with steam you get tallow, a mixture of fats, one of which is glyceryl tristearate,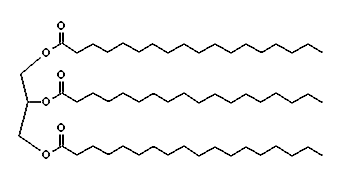
a tri-glyceride containing three stearic acid molecules attached to a glycerine molecule. When you boil glyceryl tristearate in lye (sodium hydroxide), you get sodium stearate and glycerine. When you remove the glycerine, you get soap. The sodium end of the molecule attracts water. The long hydrocarbon chain at the other end attracts oils and fats.
Uses
Soap works by helping to break fat and oil droplets into small pieces. The pieces are coated with the soap, with the hydrocarbon chains attached to the fat, leaving the sodium ends dangling in the water. The oils are now completely surrounded by water, instead of being attached to skin or clothing, and so they wash away in the rinse.sodium stearate: InChI=1/C18H36O2.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20;/h2-17H2,1H3,(H,19,20);/q;+1/p-1/fC18H35O2.Na/q-1;m
stearic acid: InChI=1/C18H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h2-17H2,1H3,(H,19,20)/f/h19H
By Simon Quellen Field