Ingredients --
Sodium nitrite
Chemical Formula: NaNO2
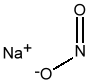
Synonyms
Nitrous acid sodium saltDescription
White or yellowish powder.Uses
Sodium nitrite is used to fix the colors in preserved fish and meats. It is also important (along with sodium chloride) in controlling the bacterium Clostridium botulinum, which causes botulism. Lunch meats, hams, sausages, hot dogs and bacon are usually preserved this way. In medicines, it is a vasodilator, intestinal relaxant, bronchodilator, and an antidote to cyanide poisoning, and hydrogen sulfide poisoning. Sodium nitrite is produced in the human body by the action of saliva on sodium nitrate, and is important in controlling bacteria in the stomach, to prevent gastroenteritis. The body produces more sodium nitrite than is consumed in food. Sodium nitrite can react with proteins in the stomach or during cooking (especially in high heat, such as frying bacon) to form carcinogenic N-nitrosamines. To prevent this, ascorbic acid or erythorbic acid are now added to the cured meat. During the curing process, some of the nitrites are converted to nitric oxide. This combines with the myoglobin proteins in the muscle of the meat to form the deep red nitric oxide myoglobin, which turns bright pink during the smoking process. This is why ham is pink. Nitrites prevent foods from getting rancid. Besides the taste issues, nitrites prevent the formation of toxic maldonadehyde, which is formed as foods get rancid.sodium nitrite: InChI=1/HNO2.Na/c2-1-3;/h(H,2,3);/q;+1/p-1/fNO2.Na/q-1;m
sodium nitrate: InChI=1/NO3.Na/c2-1(3)4;/q-1;+1
By Simon Quellen Field