Ingredients --
Para Amino Benzoic Acid (PABA)
Chemical Formula:
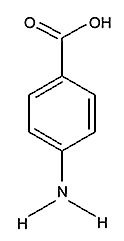
Synonyms
p-aminobenzoic acid, 4-aminobenzoic acid, Ethoxylated ethyl 4-aminobenzoic acid (PEG-25 PABA),Description
Para-amino benzoic acid (PABA) is considered to be in the B-complex vitamin family. The human body can make it from folic acid, since PABA forms the middle part of that vitamin: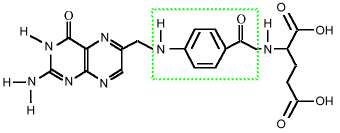
folic acid, showing PABA inside This means that in the strict sense, it is not a vitamin, since the body can make it. In this sense, however, Vitamin A is not a vitamin, since the body makes that from beta carotene. Bacteria in the intestines produce folic acid if there is PABA present. In fact, since PABA is needed by microbes, but is not strictly necessary for humans, a class of drugs that simulate PABA can fool the microbes into trying to use then instead of PABA, which causes them to die. This class of drugs is the sulfa drugs, and that is why people taking them are advised not to take PABA at the same time.
Uses
PABA is taken orally in vitamin supplements. However, its most widely known use is as a sunscreen. Taking it orally will not protect from the sun, as the sunscreen function is purely a matter of PABA acting as a dye that absorbs ultraviolet light. You need to paint it on the skin for it to block the sun. PABA is acidic, and can sting if it gets in the eyes. Some people are sensitive to PABA when it is applied to the skin. PABA also darkens to stain clothing. For these reasons modifications to PABA have become popular as sunscreens. Reacting PABA with long chain alcohols to form PABA esters like polyethylene glycol 25 PABA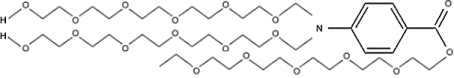
PEG-25 PABA ester can eliminate the irritation and staining problems. Other PABA esters are glycerol PABA, padimate A, and padimate O.
Chemistry lesson
Molecules absorb light when the light's wavelength is just the right size to cause electrons in the molecule to vibrate in time with the light. The electrons resonate in the molecule. In some molecules, the electrons are not bound to a single atomic nucleus, but instead roam free across several nuclei, in what are called resonance bonds. You may notice that sometimes a six-sided ring is shown with alternating double and single bonds, while at other times it is shown with a circle inside. Both forms are showing the same thing. The circle just draws our attention to the fact that the bonds don't really alternate between double and single, but they are more like 1½ bonds -- the electron spends half of the time in one place, and half of the time in the other, on average. In para amino benzoic acid, there is another resonance structure right next to the six-sided ring. It is a carboxyl group, shown with a single bond between carbons, and a double bond between the carbon and the oxygen. This is also a place where the electron can bounce around between the three nuclei. It is even possible for an electron to move back and forth from one end of the molecule to another, across all of the resonance bonds. This lets it slosh back and forth, like water in a bathtub. In the case of para amino benzoic acid, the rhythm of the sloshing matches the frequency of UV-B light. The electron can move in time with the light wave, and absorb its energy. The energy is later released as photons of longer wavelength light, such as heat. Knowing this, we can design other molecules that have resonance structures that will catch UV-B rays. One such molecule is benzophenone, and a derivative called benzophenone-3, also known as oxybenzone.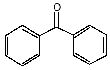
benzophenone
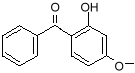
benzophenone-3 (oxybenzone) Notice that both molecules have the six-sided ring (called a benzine ring, and a carboxyl group (the carbon-oxygen double bond). These molecules also protect against UV-A. You can see that they have longer chains of resonance bonds, and so will resonate at longer wavelengths. Getting more creative with our chemistry, we can add other desirable features to our molecules. We can add long chains of hydrocarbons to make it mix better with suntan oils, and spread easily on the skin. We can modify the resonance bonds a little bit, to get a broader absorption range, into the longer wave UV-A region. Such a designer molecule is octocrylene and a similar one known as avobenzone
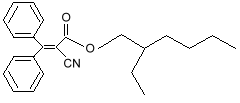
octocrylene Other molecules with the benzine ring and carboxyl group also have side chains added to make them better sunscreens. Two popular ones are 2-Ethylhexyl Salicylate
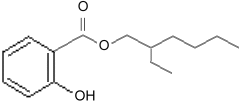
2-Ethylhexyl Salicylate and Homomethyl salicylate, also known as homosalate
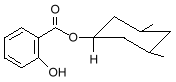
homomethyl salicylate Other salicylates used as sunscreens are 4-isopropylbenzyl salicylate, and triethanolamine salicylate, which goes by the tradename Trolamine Salicylate. Another class of UV-B absorbers is the cinnamates.
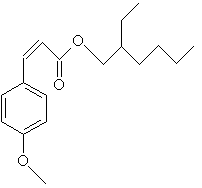
octyl methoxycinnamate These molecules are not water soluble, and stick to the skin well, and are good for water resistant sunscreens. Others in this family are Isopentenyl-4-methoxycinnamate also known as Isoamyl 4-methoxycinnamate, and Cinoxate. As you can see, there are many molecules that will absorb ultraviolet light. There are many more in use than I have described here. But they all have resonance bonds in common.