Ingredients --
Benzoyl peroxide
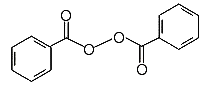
Chemical Formula: (C6H5CO)2O2
Synonyms
Description
White or off-white needle shaped water-soluble crystals
Uses
Benzoyl peroxide is used as a bleaching agent in wheat flour,
but its more familiar use is as an acne medication.
Benzoyl peroxide has several benefits as an acne medication.
It causes dead skin cells to slough off, like
salicylic acid
and
resorcinol.
It kills the bacterium
Porpionibacterium acnes that
causes acne pimples.
It has an anti-inflammatory effect that reduces pimples and
the pain they cause.
It is an anti-oxidant, reducing the oxygen free radicals that
can damage skin and DNA. At the same time, it is a bleaching
agent, oxidizing dyes to make them colorless.
It eliminates fatty acids on the skin.
Chemistry lesson
A
peroxide is a compound that has an oxygen-oxygen bond.
That is, it has two oxygen atoms bound together.
Look at the structural formula at the top of the page.
The molecule is symmetrical, with two identical parts joined
at their oxygen atoms with an oxygen-oxygen bond.
A familiar peroxide is
hydrogen peroxide.
It has two oxygen atoms bound together, and each of those
atoms in turn it bound to a hydrogen atom.
Peroxides make good bleaches. The oxygen atoms combine with
dyes to make the dyes colorless. This process (called
oxidation)
can kill germs.

