dideoxyadenosine
 PubChem Notes:
PubChem Notes:
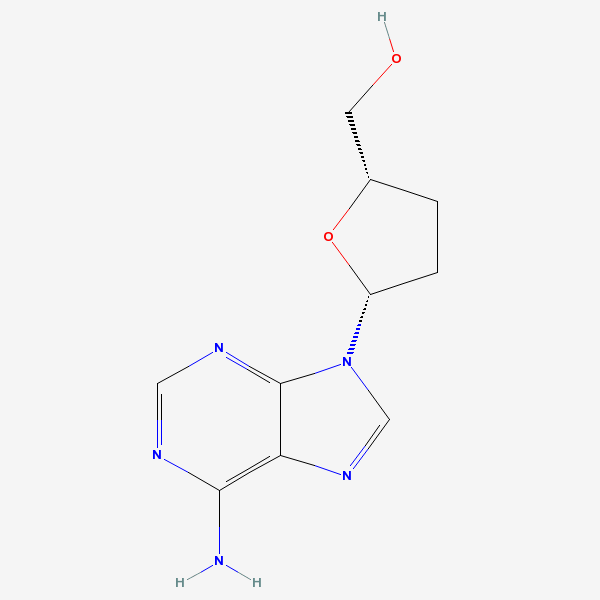
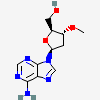
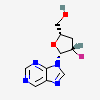
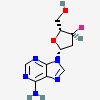
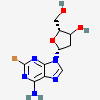
Dideoxyadenosine A dideoxynucleoside compound in which the 3'-hydroxy group on the sugar moiety has been replaced by a hydrogen. This modification prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains. The compound is an inhibitor of HIV replication, acting as a chain-terminator of viral DNA by binding to reverse transcriptase. Its principal side effect is nephrotoxicity. In vivo, dideoxyadenosine is rapidly metabolized to DIDANOSINE (ddI) by enzymatic deamination; ddI is then converted to dideoxyinosine monophosphate and ultimately to dideoxyadenosine triphosphate, the putative active metabolite.
Molecular Formula:
C10H13N5O2
InChI: InChI=1/C10H13N5O2/c11-9-8-10(13-4-12-9)15(5-14-8)7-2-1-6(3-16)17-7/h4-7,16H,1-3H2,(H2,11,12,13)/t6-,7+/m0/s1/f/h11H2
InChIKey: InChIKey=WVXRAFOPTSTNLL-JANDEZSGDA
SMILES: C1CC(OC1CO)N2C=NC3=C2N=CN=C3N
Names:
ADENOSINE, 2',3'-DIDEOXY-
BRN 0619924
CCRIS 428
ddAdo
dideoxyadenosine
DRG-0039
EINECS 223-853-2
NSC 98700
NSC-98700
2',3'-Dideoxyadenosine
[(2S,5R)-5-(6-aminopurin-9-yl)oxolan-2-yl]methanol
Registries:
PubChem CID 20039
PubChem ID 162883
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|