Paediathrocin
 PubChem Notes:
PubChem Notes:
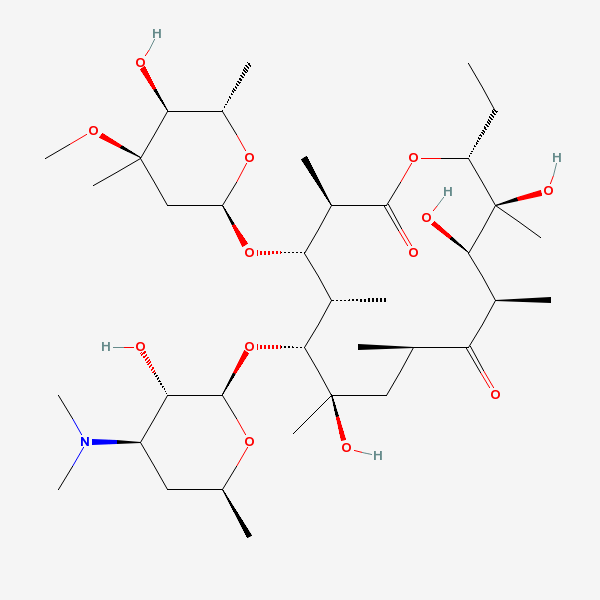
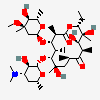
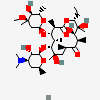
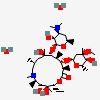
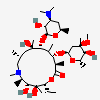
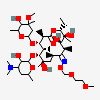
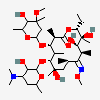
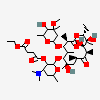
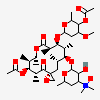
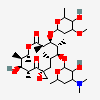
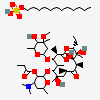
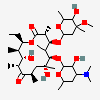
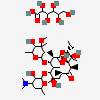
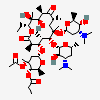
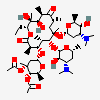
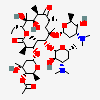
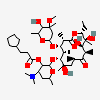
Erythromycin A bacteriostatic antibiotic macrolide produced by Streptomyces erythreus. Erythromycin A is considered its major active component. In sensitive organisms, it inhibits protein synthesis by binding to 50S ribosomal subunits. This binding process inhibits peptidyl transferase activity and interferes with translocation of amino acids during translation and assembly of proteins.
Molecular Formula:
C37H67NO13
InChI: InChI=1/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19+,20+,21+,22-,23+,24-,25-,26+,28+,29+,30-,31+,32-,34-,35-,36-,37-/m1/s1
InChIKey: InChIKey=ULGZDMOVFRHVEP-YEPXRZJNBT
SMILES: CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O
Names:
Abomacetin
Benzamycin
Dumotrycin
Endoeritrin
Eritomicina
Eritrocina
Eritromicina
Erythromycine
erythromycin
Paediathrocin
(3R,4S,5R,6R,7R,9R,11R,12R,13S,14R)-6-[(2R,3S,4R,6S)-4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione
Registries:
PubChem CID 8233
PubChem ID 151369
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|






















