|
 |
 |
 |

|
 |

|
Surfactants: the ubiquitous amphiphilesThe surfactant industry is a huge and dynamic business, and soap is just the start, says Tony HargreavesMost familiar of all surfactants is soap, a simple substance which, in water, clearly demonstrates two effects. It produces foam due to its action at the air–water interface, and it makes the grease transfer from grubby hands into the soapy water as a result of its activity at the water–oil (grease) interface. However, soap was probably not the first surfactant in the service of humankind. Many plants produce significant quantities of saponins (steroid or triterpenoid glycosides) which have surfactant properties. One such plant is the soapwort Saponaria officianalis whose foliage yields a glycoside capable of wetting, foaming and grease dispersion – the very qualities that we recognise in a modern detergent. It is likely that the saponins provided our ancestors with our first useful surfactants. These natural glycosides are still in use today for specialised processes such as washing delicate fabrics.
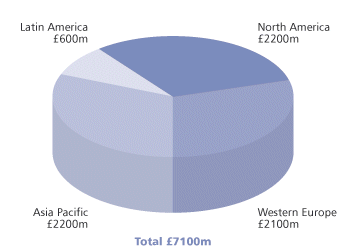
Modern surfactants, however, are of many different chemical types and do far more than produce foams and disperse grease. The global surfactant industry is a multi-billion pound business (Fig 1), with markets everywhere from household detergents to explosives (Table 1). Surfactants (surface active agents) can be broadly defined as compounds which, when dissolved in water, concentrate at surfaces (interfaces) such as water–air or water– oil. The interfacial activity of these substances, which can be explained in terms of their molecular structure, gives rise to a wide range of surface chemistry functions: wetting, emulsifying, solubilising, foaming/defoaming, rheology-modifying, antistatic, ‘glossing’, lubricity and surface conditioning.
Seldom are surfactants on their own put directly into use. In the area of household cleaning preparations, the surfactant is normally blended with a range of ingredients such as other surfactants, thickeners, foaming or defoaming agents, alkalis/salts, chelating agents and so on. This is the province of specialist formulators such as Colgate Palmolive, Unilever, and Procter & Gamble. Generally, these modern formulated products rely on man-made surfactants but there are exceptions. Certain foods, in particular, include natural surfactants such as lecithin, an emulsifier in chocolate and ice cream manufacture. Nature also relies heavily heavily on surfactant chemistry: our liver produces surfactants, the bile acids; our lungs use surfactants to maximise the efficiency of gas exchange across the air–water interface; and in every cell in every organ in our bodies there is a complex membrane that functions due to surfactant chemistry.
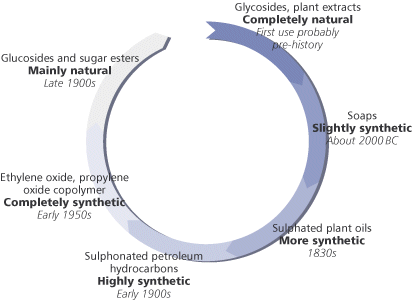
Surfactants in antiquityPeople first began to make surfactants, namely soap, in about 1500 BC, but soap-like substances have been found dating back to 2800 BC. Soap was, and still is, made by the alkaline hydrolysis of animal fats or vegetable oils – a process known as saponification. Soap is the most widely used single surfactant, accounting for around 30 per cent of the current surfactant market. Moving on from soaps – and into the 19th century – the next surfactants to be developed were the sulphates and sulphonates of vegetable oils. The reaction of castor oil with sulphuric acid is a classic example from the late 1800s. In this reaction the product is a mixture of sulphates and sulphonates which, after neutralisation with sodium hydroxide, give a product known as Turkey Red oil useful in the dyeing of linen.
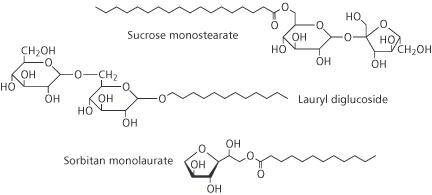
Later, the development of sulphonation and sulphation processes using other oils as reactants led to a move away from natural and renewable plant oils and animal fats to the sulphonation of petroleum products. The introduction of alkyl benzene sulphonatis (ABSs), for example, was brought about by nucleophilic substitution in the benzene ring using oleum (H2S2O7) or sulphur trioxide. The ABSs made a major contribution in changing the traditional soap powders to detergent powders for household laundry. One well-known example in the 1950s was the change in the composition of Lever Brothers’ Persil, where the soap in the original formulation (PERborate+SILicate+soap) was replaced by ABS. Other brands of washing powders followed suit and the word detergent is now a part of everyday vocabulary. Modern detergent powders now typically contain linear alkylbenzene sulphonates (LABSs), which biodegrade more quickly than the original ABSs. Progress was not confined to the sulphonation of different oils, but was soon accompanied by ethoxylation, in which a few or many ethylene oxide (EO) molecules react with a fatty alcohol – which may be synthetic or plant derived – to make the surfactant molecule. Thus, alcohol ethoxylates, alcohol ether sulphates and alkyl phenol ethoxylates became available. Along with the development of ether technology came the polymerisation of ethylene oxide with propylene oxide (PO) to give EO–PO copolymers – surfactants that are totally reliant on petrochemicals as raw materials. But things may slowly be beginning to turn back around. Current pressure to move away from non-renewable petroleum feedstocks and towards plants as sources of raw materials has led to a lot of effort on developing surfactants from oleochemical feedstocks. Many recently-developed surfactants are an attempt to satisfy the modern consumers’ desire for products to be ‘more natural’. Most important amongst these are surfactants derived from the carbohydrates sorbitol, sucrose, glucose and from plant oils such as coconut or palm kernel. Thus we have: sorbitan esters, sucrose esters, alkyl polyglucosides (AGs), alkyl glucamides (Box 2). Sorbitan esters are used as emulsifiers in cosmetics and the sucrose esters in food manufacture. The alkyl polyglucosides find application as detergents rather than as emulsifiers and are making inroads into some everyday products.
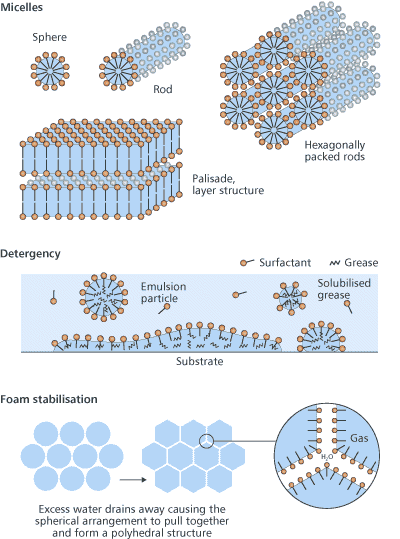
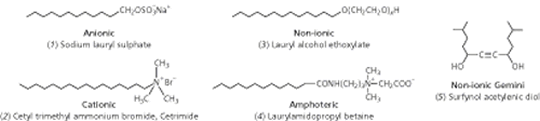
How surfactants workA look through the surfactant literature presents us with a huge number of different surfactants, along with an even greater number of names. At the core of all this is some relatively simple chemistry. All these surfactant molecules have in common the same basic molecular structure – a hydrophile attached to a lipophile – and it is this nature that makes them adsorb at surfaces. The hydrophile is attracted to water in preference to lipid-like substanoes (hydrocarbons such as oil and grease) whereas the lipophile is attracted to these in preference to water. Other terms are sometimes used, for example, a hydrophile is a lipophobe and a lipophile is a hydrophobe. However, the use of ‘phobe’ is unfortunate because it implies repulsion whereas the true situation is one of only feeble attraction. Paraffin wax is lipophilic but it does not repel water, as is demonstrated by the fact that droplets of the liquid cling, somewhat precariously, to the underside of a wax block – the water must be attracted to the wax. It is the amphiphilic nature of surfactant molecules that makes them bifunctional. This can be seen when oil/grease and water come together. No matter how much energy is expended in getting them to mix, the oil and water will always separate into two distinct phases. The intermolecular forces between water molecules and between oil molecules are stronger than the forces between water and oil molecules. Added surfactant molecules adsorb at the oil–water interface, where they orient themselves such that the hydrophile is in the water and the lipophile is in the oil. With a little agitation the oil becomes dispersed in the water and the surfactant acts as an emulsifying agent. Types of surfactantsLipophiles are usually similar from one surfactant to another but hydrophiles show a range of chemical types and this is the basis for surfactant classification: anionic, cationic, non-ionic and amphoteric (Fig 2).
Anionic surfactants, which include soap, are the most widely used for cleaning processes because many are excellent detergents. In anionic surfactants the hydrophile comprises some highly electronegative atoms, making these molecules strongly polar. The counterion is usually a small cation such as sodium but occasionally may be a larger cation such as ammonia or amines. Cationic surfactants, in contrast, comprise a long chain hydrocarbon as the lipophile with a quaternary amine nitrogen as hydrophile, and a halide ion as counterion. An important property of cationics is that they are attracted to surfaces carrying a negative charge, upon which they adsorb strongly. Proteins and synthetic polymers can thus be treated with cationics to provide desirable surface characteristics. For example, hair conditioners and fabric softeners are cationic surfactants. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||