Ingredients --
Isobutane
Chemical Formula: C4H10
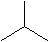
Isobutane
Synonyms
ButaneDescription
Colorless, odorless gas.Uses
Isobutane is used in cigarette lighters and camp stoves as a fuel. It is easily liquified under pressure, and the liquid becomes a gas immediately when the pressure is released. Isobutane is also used as a propellant in some hair sprays and in spray breath fresheners.Chemistry lesson
Isobutane is a compund in a class of chemicals called alkanes. Alkanes are chains of carbon atoms where each carbon atom has as many hydrogen atoms attached as possible. This means that all of the bonds between carbon atoms are single bonds (no double bonds). Such a molecule is said to be saturated. The simplest alkane has only one carbon atom. It is called methane.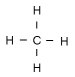
Methane CH4 We show it here with all of the hydrogens showing. Normally, in pictures of the structural formulas in organic chemistry, we don't show carbon atoms or hydrogen atoms, because the picture would just get too crowded. A carbon atom is implied whenever a line ends, or whenever a line joins another. Hydrogens are assumed to connect to any carbon that has a free bond (carbon can form four bonds, so if only three are showing, the other is attached to a hydrogen).

Ethane C2H6 Using this convention, ethane is just a line. There is a carbon at each end, and each carbon has three bonds left, so there are six hydrogens in the molecule.

Propane C3H8 Propane is a bent line. There is a carbon at each end, and one in the middle, at the bend. Each carbon at an end has three bonds, and the carbon in the middle has only two bonds left, so we have eight hydrogens in the molecule.

Butane C4H10 Butane has four carbons. With four carbons, we suddenly have two different ways the carbons can link up. They can be all in a row, like the molecule shown above, or one carbon can be in the middle, bonding to three carbons and a hydrogen.

Isobutane C4H10 We call the one with all the carbons in a row butane, and the form with one carbon in the middle isobutane. The iso is short for isomer, which means a molecule with the same atoms, but arranged in a different way.

Pentane C5H12 When we get up to five carbons, there are three different ways to arrange them. We call the molecule pentane when the carbons are all in a row.
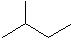
2-methylbutane C5H12 When molecules start to get complicated like this, we name them carefully, according to some rules. One rule is that we look for the longest unbranched alkane in the molecule. In this molecule, it is butane (4 carbons). Then we describe the branch. In this case it is methane (one carbon). Then we describe where the branch is attached. In this case it is attached to the second carbon in butane. The name is thus 2-methyl-butane.

2,2-dimethylpropane C5H12 The other way 5 carbon atoms can be arranged in an alkane is to attach two of them to the middle carbon atom in propane. We have 2 methanes, and a propane, and both methanes are attached to the second carbon in the propane. Thus we call it 2,2-dimethyl-propane. Up until we got to pentane, all of these alkanes were gasses. Pentane is a liquid. The longer the chain of carbons gets, the harder it is for one of the molecules to escape into the air.
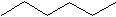
Hexane C6H14 Hexane is next. There are many ways to put 6 carbons together, so we will only show two forms here. The first is simply the carbons all in a row.
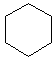
Cyclohexane C6H12 Another way to put 6 carbons together is to attach them in a ring. The result is not the same as hexane, because the two hydrogens at the end of hexane are missing. The ring form is called cyclohexane. While three carbons could have formed a three sided ring, the bonds would be very strained, and the molecule would be unstable. Likewise, four and five carbon rings can form, but they are also less stable, and break down into the other forms. But with six carbons, the molecule is quite stable, and stays in a ring easily.

Heptane The simple chain made of seven carbons is called heptane.

Octane When eight carbons are in a chain, we get octane. A mixture of liquid alkanes we call gasoline burns in our automobiles. If the mixture has a lot of light-weight molecules that evaporate easily, it can ignite too readily in the engine, causing premature ignition, or "knocks and pings". If the gasoline has more of the longer molecules, it burns more slowly, and only ignites when the spark plug fires. We call these gasolines "high octane". Alkanes keep going up in size. Once we get up to C18H38 they are solids. The familiar white solid parafin used for making candles is made up of solid alkanes. It is not really a wax, as waxes are more complicated molecules.
isobutane: InChI=1/C4H10/c1-4(2)3/h4H,1-3H3
methane: InChI=1/CH4/h1H4
ethane: InChI=1/C2H6/c1-2/h1-2H3
propane: InChI=1/C3H8/c1-3-2/h3H2,1-2H3
butane: InChI=1/C4H10/c1-3-4-2/h3-4H2,1-2H3
pentane: InChI=1/C5H12/c1-3-5-4-2/h3-5H2,1-2H3
hexane: InChI=1/C6H14/c1-3-5-6-4-2/h3-6H2,1-2H3
cyclohexane: InChI=1/C6H12/c1-2-4-6-5-3-1/h1-6H2
heptane: InChI=1/C7H16/c1-3-5-7-6-4-2/h3-7H2,1-2H3
octane: InChI=1/C8H18/c1-3-5-7-8-6-4-2/h3-8H2,1-2H3
By Simon Quellen Field