Ingredients --
How to Read Structural Formulas
Simple Formulas
Some formulas, such as the formula for table salt, are very simple. We don't need a picture, just a description of the two elements that make it up, sodium and chlorine. The formula is: NaCl Other compounds are also fairly simple, although they may contain more elements. For example, phosphoric acid, a common ingredient in soft drinks (used to provide tartness) is: H3PO4 This says it is made up of three hydrogen atoms, one atom of phosphorus, and four atoms of oxygen. A molecule you probably already know is water: H2OFull Structural Formulas
For some compounds, it is especially useful to know the shape of the molecule. While this is critically important in large molecules made with a backbone of carbon, called organic molecules, it is often interesting in simpler molecules. The formula given above for water might cause someone to think that a hydrogen atom was attached to another hydrogen atom, and then an oxygen atom was attached to them: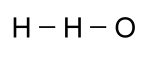 That would be wrong, however, since a hydrogen atom can only
form a bond with one other atom. Moreover, oxygen can bond
with two other atoms, so a better picture would look like this:
That would be wrong, however, since a hydrogen atom can only
form a bond with one other atom. Moreover, oxygen can bond
with two other atoms, so a better picture would look like this:
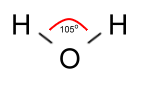
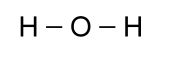 As it turns out, however, the angle between the bonds is not 180°
as that picture would make it seem, but 105°, which is important
in understanding some curious facts about water:
As it turns out, however, the angle between the bonds is not 180°
as that picture would make it seem, but 105°, which is important
in understanding some curious facts about water:
 The electrons that the hydrogens share with the oxygen are located
between the hydrogens and the oxygen. This leaves the hydrogens
with a little bit of a positive charge, and the oxygen with a
little bit of a negative charge. This polar arrangement
means that the molecules prefer to align in certain ways, because
the positive sides attract the negative sides. This gives water
its surface tension, and explains why ice crystals arrange in
hexagons, and take up more space than liquid water, making ice
less dense than water, so it floats. It also explains how water
molecules can dissolve other polar molecules like table salt. The
water molecules surround the positive sodium ions and the negative
chlorine ions, and prevent them from getting back together.
The electrons that the hydrogens share with the oxygen are located
between the hydrogens and the oxygen. This leaves the hydrogens
with a little bit of a positive charge, and the oxygen with a
little bit of a negative charge. This polar arrangement
means that the molecules prefer to align in certain ways, because
the positive sides attract the negative sides. This gives water
its surface tension, and explains why ice crystals arrange in
hexagons, and take up more space than liquid water, making ice
less dense than water, so it floats. It also explains how water
molecules can dissolve other polar molecules like table salt. The
water molecules surround the positive sodium ions and the negative
chlorine ions, and prevent them from getting back together.
Simplified Structural Formulas
Earlier, we said that hydrogen atoms can only bond with one other atom. This is because they only have one electron to share. The element carbon has four electrons it can share easily, and so it can bond to four different atoms at a time. Because carbon is so versatile, it can form very complex molecules. These complex molecules are what led to life on this planet, and living things are primarily made of large molecules with a backbone of carbon. Simple carbon compounds such as methane are often written using non-structural formulas, such as: CH4 or the structural formulas we have just discussed: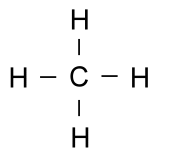 But when the molecules start getting larger, all those letters for carbon
and hydrogen start to clutter up the page, making it hard to see detail.
Since we know that carbon has four bonds to fill, and that hydrogen
is the most likely atom to be attached to carbon in an organic
molecule, we can invent a shorthand notation that is easier to read
and draw.
In our shorthand, we will assume that any vertex between two lines
contains a carbon atom, unless we specify otherwise. If there are
several carbons in a row, we will not just draw a long line, but we
will make the lines join at angles, so we can count the carbons if
need be.
We also will say that any carbon that has fewer than four lines from
it will be assumed to have a hydrogen atom filling all the remaining
bonds.
Thus the molecule propane (C3H8),
which has three carbons in a row,
and all of the remaining bonds filled with hydrogen:
But when the molecules start getting larger, all those letters for carbon
and hydrogen start to clutter up the page, making it hard to see detail.
Since we know that carbon has four bonds to fill, and that hydrogen
is the most likely atom to be attached to carbon in an organic
molecule, we can invent a shorthand notation that is easier to read
and draw.
In our shorthand, we will assume that any vertex between two lines
contains a carbon atom, unless we specify otherwise. If there are
several carbons in a row, we will not just draw a long line, but we
will make the lines join at angles, so we can count the carbons if
need be.
We also will say that any carbon that has fewer than four lines from
it will be assumed to have a hydrogen atom filling all the remaining
bonds.
Thus the molecule propane (C3H8),
which has three carbons in a row,
and all of the remaining bonds filled with hydrogen:
 becomes the much simpler looking picture:
becomes the much simpler looking picture:
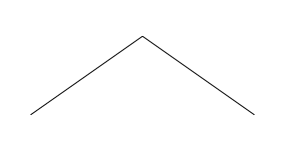 There is a carbon at each end, and one in the middle. The carbons
at each end have three remaining bonds, which are filled with three
hydrogens. The carbon in the middle has only two bonds left, so
there are two hydrogens there.
Now for simple molecules like propane, this is not much of an
improvement. It is easier to draw, but the reader has to do some
thinking to do to figure out at first what the molecule is.
However, with larger molecules, the simplification really helps
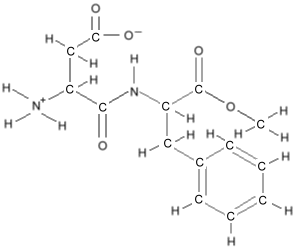
to keep the picture uncluttered. Consider the molecule for
aspartame, which would look like
this:
There is a carbon at each end, and one in the middle. The carbons
at each end have three remaining bonds, which are filled with three
hydrogens. The carbon in the middle has only two bonds left, so
there are two hydrogens there.
Now for simple molecules like propane, this is not much of an
improvement. It is easier to draw, but the reader has to do some
thinking to do to figure out at first what the molecule is.
However, with larger molecules, the simplification really helps
to keep the picture uncluttered. Consider the molecule for
aspartame, which would look like
this:
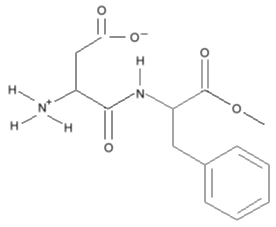
 but simplifies to this:
but simplifies to this:
Three dimensional Structural Formulas
Sometimes it is important to know the three dimensional structure of a molecule. In glucose for example,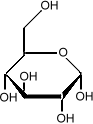 it is important to know which side of the molecule the various
hydroxyl groups (the OH sub-units) are. Flipping one
of them from the bottom to the top changes the sugar from
glucose to galactose:
it is important to know which side of the molecule the various
hydroxyl groups (the OH sub-units) are. Flipping one
of them from the bottom to the top changes the sugar from
glucose to galactose:
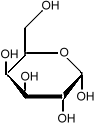 which is much less sweet than glucose.
We indicate that some parts of the molecule are closer to the
reader by making the lines darker.
which is much less sweet than glucose.
We indicate that some parts of the molecule are closer to the
reader by making the lines darker.
By Simon Quellen Field