Week 4: The Spectra of Conjugated Dyes and Investigation of Beer's Law |
Overview
Getting Started
Techniques
Procedure
FAQ
Full Lab Manual
Introduction & Goals
Chemistry & Background
Key Questions
Prelab Problems
Safety
Procedure
In Your Write-up
Experiments Index
ChemLab Home
Color and Light
The color of most objects depends upon the interaction between visible light and the electrons of atoms or molecules that make up the object. It is usually the result of a dynamic process on the molecular level: the absorption of light and the resulting change of a molecule's quantized energy. The object absorbs certain wavelengths of white light, and we see what is left over. The particular wavelengths of light that a given substance absorbs determine the color we perceive and depend on the energy levels of the molecules or atoms that make up the substance. This absorption coloration mechanism is responsible for the colors of grass, blood and carrots, but not of the sky. The sky's color is due to selective scattering of different wavelengths of sunlight by the molecules of nitrogen and oxygen in the atmosphere.
Visible light is the very small portion of the electromagnetic spectrum that human eyes are sensitive to. Light can be described as oscillating electric and magnetic fields, as can radio waves, microwaves, infrared radiation, ultraviolet light, and x-rays. Visible light differs from these other types of light because it has a range of wavelengths that our eyes can detect. These visible wavelengths match the differences between quantized energy levels of the detection molecules in the retina of the human eye. Thus, the first step in the perception of color also involves the absorption of visible light by molecules, in the retina of the perceiver's eye.
The perceived color of an object has a complementary relationship with the color of the visible light absorbed. First consider white light, a mixture of all visible wavelengths, impinging on a colored object. The object absorbs some wavelengths of the light; exactly which ones depends on the component molecule's energy levels. The wavelengths of light that are not absorbed are transmitted or reflected to the observer's eye. A substance that appears blue is transmitting or reflecting blue light to the eye and absorbing other colors of the white light that are not blue. There are two ways for a material to produce the perception of a particular color. One is to absorb all wavelengths of visible light, aside from the perceived color. For our blue example, the material would absorb red, orange, yellow, and violet light. The absorption spectrum would show high absorbance of all visible wavelengths, besides blue. The transmission or reflectance spectrum would have a maximum at a wavelength corresponding to blue light. The second way to create the perception of blue is for a material to have a strong absorbance of the opposite or complementary color of light. A color wheel, shown below, illustrates the approximate complementary relationship between the wavelengths of light absorbed and the wavelengths transmitted or reflected. Your textbook has a color version of this color wheel that can help you understand this complementary relationship. In the example of a blue substance, there would be a strong absorbance of the complementary color of light, orange. For this case, the absorption spectrum of a blue solution would have a maximum absorbance at a wavelength corresponding to orange light.
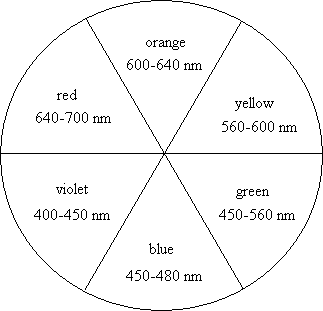
Color wheel with approximate wavelength values
for different color light.
Ephoton = h = =  Emolecule =
Eupper state - Elower state Emolecule =
Eupper state - Elower state |
(1) |
So to understand the color of an object, which arises from its absorption of light, we must know the array of possible energy levels of its molecules. In general these energy levels include states of quantized rotational, vibrational, and electron energy. These correspond to rotation of the entire molecule, vibration of the chemical bonds within the molecule, and changes in the electron configuration of the molecule. With rare exceptions, colored substances that absorb photons in the visible region of the spectrum undergo a transition that changes the electron energy levels of the molecule. The photon of visible light is absorbed and excites a molecule from its lowest-energy or ground electron configuration to a higher-energy electron configuration. Transitions between electron configurations, or electronic states, are responsible for the majority of the colors we see in the natural world.
You may be wondering what happens to the energy of the photon, after the molecules of a colored substance absorb it. Most typically, the molecule quickly returns to its ground electron configuration, with the extra energy imparted by the photon converted to vibration and rotation of the molecule. The visible light absorbed is thus converted to vibration and rotation, which are the molecular basis of heat. This heat will raise the temperature of the material. Here is a familiar example: A white material, which reflects all visible wavelengths, does not heat up in the sun as much as a black material, which absorbs all visible wavelengths. In this process, first the visible photons are absorbed, causing the electrons in the black material to be excited. Then the electrons return to their ground state configurations and the extra energy moves to vibrational and rotational motion, causing the temperature of the material to increase.
The Quantum-Mechanical Particle-in-a-Box
Many dye molecules are members of a special group for which a very simple quantum-mechanical model can predict the wave functions and energy levels of the electrons responsible for the visible wavelength transitions and therefore the color of the dye. We will apply the particle-in-a-box model, discussed in lecture, to the electrons in a dye molecule with a box-like structure. Because of the arrangement of the electrons in these dyes, some of the electrons are "delocalized" or essentially free to move along the molecule's carbon-carbon chain, like a particle-in-a-box. We will begin with a review of the particle-in-a-box problem and its solution and then discuss the structure of the dyes to be investigated.
deBroglie postulated that all moving particles have an associated wavelength that in certain situations (e.g. diffraction) governs the behavior of the particle. For a particle moving in a region of constant potential energy, the deBroglie condition is
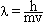 |
(2) |
This relation allows calculation of the particle's wavelength
 , given its mass, m, and
velocity, v. This equation is directly applicable to the
quantum-mechanical particle-in-a-box because the potential energy V is
constant (namely zero) within the box. The waves whose amplitude-squared
(
, given its mass, m, and
velocity, v. This equation is directly applicable to the
quantum-mechanical particle-in-a-box because the potential energy V is
constant (namely zero) within the box. The waves whose amplitude-squared
( 2) determine the
probability distribution of the electron's location
within the box are standing waves analogous to the wave motion set up on a
plucked string. The string is clamped at both ends and when plucked will
vibrate in a complex manner. However, the vibrational motion of the string
can be mathematically resolved (by Fourier analysis) into simple motions
which when added together reproduce the actual vibrational motion of the
string.
2) determine the
probability distribution of the electron's location
within the box are standing waves analogous to the wave motion set up on a
plucked string. The string is clamped at both ends and when plucked will
vibrate in a complex manner. However, the vibrational motion of the string
can be mathematically resolved (by Fourier analysis) into simple motions
which when added together reproduce the actual vibrational motion of the
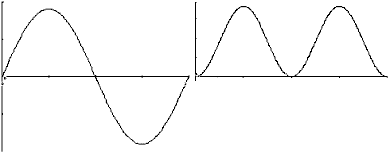
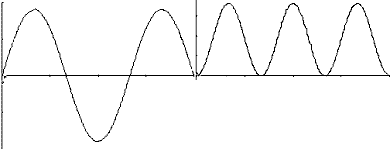
string.For a typical, well-plucked string, the main contribution to the overall vibration comes from the lowest frequency or fundamental mode. This mode can be represented by a graph that shows the string position at some instant in time:
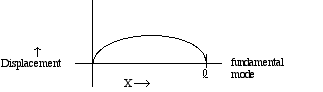

 while the wavelength for the
fundamental mode is 2
while the wavelength for the
fundamental mode is 2  ,
since only half a wave fits in between 0 and
,
since only half a wave fits in between 0 and  . Note as well that for the
first overtone, there is a node or motionless point at x equal to
. Note as well that for the
first overtone, there is a node or motionless point at x equal to  /2. Higher order overtones
have shorter wavelengths and more nodes. The allowed wavelengths which
involve displacements of zero at x = 0 and x =
/2. Higher order overtones
have shorter wavelengths and more nodes. The allowed wavelengths which
involve displacements of zero at x = 0 and x =  are 2
are 2 , 2
, 2 /2 or
/2 or  , 2
, 2 /3, 2
/3, 2 /4 or
/4 or  /2 and so on. This series may
be represented by the equation
/2 and so on. This series may
be represented by the equation
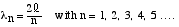 |
(3) |
Exactly the same set of wavelengths governs the behavior of a quantum particle-in-a-box. Recall that the particle has zero potential energy inside the box, but the potential goes to infinity at the edges of the box. The infinite potential energy at 0 and
 insures that the particle
cannot escape from the potential well and that the particle's wave
function
insures that the particle
cannot escape from the potential well and that the particle's wave
function 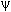 equals zero for x
< 0 and x >
equals zero for x
< 0 and x >  . The
requirement that
. The
requirement that 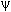 behave in
a smooth, wavelike fashion means that
behave in
a smooth, wavelike fashion means that 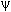 must be zero at both 0 and
must be zero at both 0 and  as well. Thus the allowed
as well. Thus the allowed
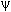 functions look just like
the graphs of the vibrational displacements of a string. The value of
functions look just like
the graphs of the vibrational displacements of a string. The value of 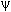 2(x) gives the
probability of finding the electron at a
particular value of x. The wave functions and
2(x) gives the
probability of finding the electron at a
particular value of x. The wave functions and 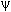 2(x) functions are
shown in the following graphs.
2(x) functions are
shown in the following graphs.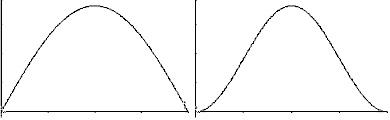
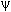 2(x) or
probablility densitity functions (right graph of each pair)
2(x) or
probablility densitity functions (right graph of each pair) for a particle-in-a-box.
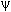 1 (quantum number
n=1), the particle has maximum probability of being at
1 (quantum number
n=1), the particle has maximum probability of being at  /2 while for a particle with
wavefunction
/2 while for a particle with
wavefunction 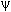 2,
the particle has zero probability of being at
2,
the particle has zero probability of being at  /2.
/2.In order to understand light absorption by electrons which behave like quantum particles in a potential box, we need to know the energy of an electron in the various states corresponding to the quantum number n = 1, 2, 3, 4, ... Because the potential energy is zero throughout the region from 0 to
 , the particle's total energy
En equals its kinetic energy. We can use the definition of
kinetic energy, KE = 1/2 mv2, and with the help of Equations
(2) and (3) we have
, the particle's total energy
En equals its kinetic energy. We can use the definition of
kinetic energy, KE = 1/2 mv2, and with the help of Equations
(2) and (3) we have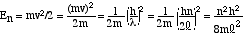 |
(4) |
Equation (4) allows us to calculate the quantized energy levels for a particle confined to a box, where its potential energy is zero inside the box and rises to infinity at the edges. The energy levels depend on the quantum number n, the length of the box
 ,
and the mass of the particle, m. The spacing between adjacent energy
levels is inversely proportional to the particle mass and the square of
the box length. Thus discrete energy levels are observable for
sufficiently light particles confined to small quarters (e.g., electrons
within atoms or molecules, with small values of m
,
and the mass of the particle, m. The spacing between adjacent energy
levels is inversely proportional to the particle mass and the square of
the box length. Thus discrete energy levels are observable for
sufficiently light particles confined to small quarters (e.g., electrons
within atoms or molecules, with small values of m 2).
2).Many molecular substances are colorless because the spacing between the highest occupied electronic energy level and the lowest unoccupied level typically are larger than the energy of a photon in the visible range. However, for sufficiently large molecules (large
 ) this energy level
separation may correspond to a visible photon, provided that there are
high-energy electrons ("valence electrons") which can travel freely over
the distance
) this energy level
separation may correspond to a visible photon, provided that there are
high-energy electrons ("valence electrons") which can travel freely over
the distance  . These
free electrons then behave approximately like the particles in our
one-dimensional box model. We will apply the energy level expression of
Equation (4) to the case of electrons that move freely in one part of a dye molecule. The
result is called the Free Electron Model and
will enable us to calculate theoretical energy levels and compare them to
the result of experiments determining the difference between a molecule's
energy levels from its absorption spectra.
. These
free electrons then behave approximately like the particles in our
one-dimensional box model. We will apply the energy level expression of
Equation (4) to the case of electrons that move freely in one part of a dye molecule. The
result is called the Free Electron Model and
will enable us to calculate theoretical energy levels and compare them to
the result of experiments determining the difference between a molecule's
energy levels from its absorption spectra.Cyanine Dyes
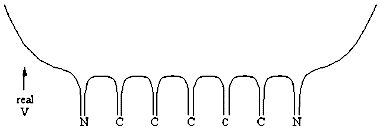
You will carry out experiments on two cyanine dyes for which the free electron model can be applied. The structural formulas of Dyes A and B are shown below. In these formulas, the lines represent chemical bonds and the corners where the lines meet, not labeled by a letter, represent a carbon atom. In between the two nitrogen atoms at the center of each dye, there are several delocalized electrons that can move freely along the central chain of the molecule, but not more than one bond length beyond the nitrogen atom. The quantized energy levels of these delocalized electrons can be calculated using the result of the particle-in-a-box problem, Equation (4). The two nitrogen atoms represent a substantial disruption of the delocalized electron system, so that the walls of the box can be estimated to fall one bond length after the nitrogen atoms. Thus, in the free electron model, the delocalized electrons will be described as particles that move freely, with zero potential energy, within the region of the "box". At the edges of the box, the free electron model assumes that the potential energy goes to infinity and the probability of finding the electrons on the other side is zero.
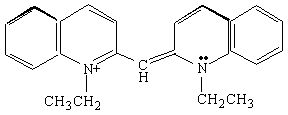
Dye B.
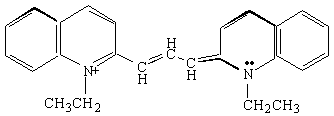
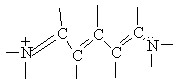
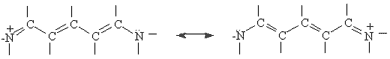
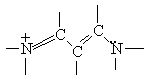
Now we will apply the particle-in-a-box result to the particular system of cyanine dye molecules. Equation (4) allows us to calculate the allowed energy levels for a particle (or several particles) in a potential energy box. We will use this equation to calculate the possible energy levels for the delocalized electrons in the part of the cyanine dye molecules where they can move freely. To calculate the energy levels, we will first substitute the mass of an electron and the length of the molecular "box" into Equation (4). To determine the appropriate value of the quantum number n, we must consider the number of delocalized electrons in each dye molecule. This will tell us how many of these energy levels are occupied by electrons. In Dye B, for example, each of the 5 carbon atoms can be thought of as contributing 1 electron, the positively charged nitrogen also contributes 1 electron, and the lone pair on the second nitrogen contributes 2 electrons; for Dye B there are 8 delocalized electrons.
Using the Pauli Principle, we place these free electrons into allowed energy levels for a particle-in-a-box (Equation (4)) two at a time with spins paired. If N equals the number of free electrons (eight for Dye B), then clearly the levels from n=1 to N/2 are occupied. For the eight delocalized electrons of the free-electron system of Dye B, the electronic configuration can be indicated schematically by the following diagram:
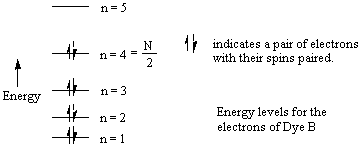
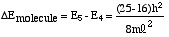 |
(5) |
Now consider the general case, in which the free electron system contains N electrons. If we let p = N/2 denote the highest occupied, free electron energy level (p=4 for Dye B), then for the lowest energy electronic transition
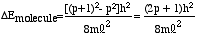 |
(6) |
The wavelength
 of the photon
absorbed in this transition is therefore given by
of the photon
absorbed in this transition is therefore given by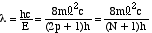 |
(7) |
where c is the speed of light and
 is
in meters, when SI units (J, kg, m, s, etc.) are chosen for m,
is
in meters, when SI units (J, kg, m, s, etc.) are chosen for m,  , c and h. The value of
, c and h. The value of  can be estimated from
experimentally determined carbon-carbon and carbon-nitrogen bond lengths.
A typical bond length for these bonds is 0.139 nm or 1.39 x
10-10 m. By counting the number of bonds and using this value,
the length of the box can be estimated. Because the electrons are able to
move past the nitrogen atoms by about one bond length, we suggest adding
an additional bond distance on either end of the nitrogen atoms, when
estimating the length. For Dye B, for example,
the total length of the box,
can be estimated from
experimentally determined carbon-carbon and carbon-nitrogen bond lengths.
A typical bond length for these bonds is 0.139 nm or 1.39 x
10-10 m. By counting the number of bonds and using this value,
the length of the box can be estimated. Because the electrons are able to
move past the nitrogen atoms by about one bond length, we suggest adding
an additional bond distance on either end of the nitrogen atoms, when
estimating the length. For Dye B, for example,
the total length of the box,  , is equal to 8 bond
lengths.
, is equal to 8 bond
lengths.Equation (7) enables us to calculate a wavelength of light required to excite an electron from the ground to first excited state, in the free electron model for conjugated dyes. This model assumes that some of the electrons in the carbon-carbon chain are free to move anywhere along the chain, but not more than a bond length beyond the nitrogen "anchors", like particles in a box. For each dye observed experimentally, Equation (7) will allow us to make a theoretical prediction of the wavelength of maximum absorbance.
If changes in only electronic energy accompany absorption of light, then Equation (7) would predict a very sharp maximum in absorption at the wavelength given. Such very sharp absorption and emission lines are indeed observed for isolated atoms, as you saw in last week's atomic spectra experiment. However, molecular substances in solution show much broader absorption bands. This is because the absorption of electronic energy by molecules is almost always accompanied by changes (which can be either increases or decreases) in vibrational and rotational energy. Since the energy levels for these vibrations and rotations of the molecule are much more closely spaced than those for electrons, there are many possible closely spaced wavelengths at which light can be absorbed, which overlap to form a broad absorption band. With some degree of arbitrariness, we shall assume that Equation (7) predicts the wavelength
 max at which each of
our dyes absorbs most strongly.
max at which each of
our dyes absorbs most strongly.Note that the free electron model should be accepted with something less than religious fervor because the particle-in-a-box theory assumes the potential energy to be constant over the entire conjugated system and rises sharply to infinity at the edges of the box.
A diagram of such a potential is shown below.
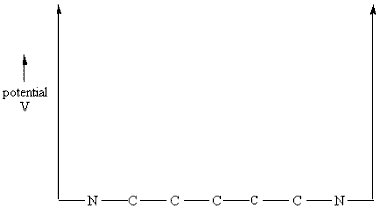
Analyzing a Dye Solution: Koolaid or Gatorade
In the second experiment of this week's lab, you will analyze a solution of drink mix to determine what food dyes are present in what quantities. Your drink solution will have an obvious color, so your goal is to determine whether the color results from a single dye or a mixture of dyes. You will then use Beer's Law to determine the concentration of all dyes in the drink solution.
Colorimetry and Beer's Law
Colorimetry involves the measurement of the amount of light absorbed by a colored sample. In the kinetics experiments of Week 1 and Week 2, you used colorimetry to monitor the change in concentration of a colored reactant with time. The introduction to Week 1, in this manual, describes the form and constants that appear in Beer's Law in detail. Recall that the equation of Beer's Law is
A = c L  |
(8) |
where
A = absorbance of the sample, also defined below in terms of light intensity
c = concentration of the absorbing species (in mol per liter units)
L = path length traveled by the light beam through the sample (in cm)
 = molar
extinction coefficient of the absorbing species (in liter mol-1
cm-1 units)
= molar
extinction coefficient of the absorbing species (in liter mol-1
cm-1 units)It is useful to interpret the Beer's Law equation in light of our previous discussion of color. The perceived color of a solution tells the observer what wavelengths of light are transmitted. A color wheel relates the color or wavelengths of transmitted light to that of the absorbed light. So for a colored solution, some wavelengths are absorbed more strongly than others. This wavelength dependence of absorbance enters Beer's Law through the extinction coefficient, which is a function of wavelength. The extinction coefficient,
 , is a
proportionality constant between absorbance and concentration, for a given
path length. A high
, is a
proportionality constant between absorbance and concentration, for a given
path length. A high  value means that a substance absorbs light strongly at the particular
wavelength. A plot of
value means that a substance absorbs light strongly at the particular
wavelength. A plot of  (or A with L and c specified) versus wavelength
(or A with L and c specified) versus wavelength (
 ) is called the absorption
spectrum of a substance. This spectrum shows which wavelengths of
light are absorbed and which are not. Recall that the perceived color may arise from
two different types of absorption spectra. A strong absorbance of the color of light
opposite the perceived color on the color wheel would give
rise to an absorption spectrum with a single band. Absorption of all
colors of light, besides the perceived color, would arise for
a substance with several visible absorption bands or for a mixture of
colored substances. Observing the color of the solution
should enable you to predict the nature of its absorption spectrum and
therefore the wavelengths of light that the sample absorbs. Measuring
absorbance vs. wavelength, or the absorption spectrum, will enable you to
distinguish between the two possible absorption spectra you would predict
for a colored solution.
) is called the absorption
spectrum of a substance. This spectrum shows which wavelengths of
light are absorbed and which are not. Recall that the perceived color may arise from
two different types of absorption spectra. A strong absorbance of the color of light
opposite the perceived color on the color wheel would give
rise to an absorption spectrum with a single band. Absorption of all
colors of light, besides the perceived color, would arise for
a substance with several visible absorption bands or for a mixture of
colored substances. Observing the color of the solution
should enable you to predict the nature of its absorption spectrum and
therefore the wavelengths of light that the sample absorbs. Measuring
absorbance vs. wavelength, or the absorption spectrum, will enable you to
distinguish between the two possible absorption spectra you would predict
for a colored solution.Beer's Law is useful in the quantitative analysis of solutions containing colored compounds, like Koolaid or Gatorade. At a particular wavelength, Beer's Law can be used to relate absorbance and concentration. Since A = c L
 , a plot of absorbance vs.
concentration, for fixed
, a plot of absorbance vs.
concentration, for fixed  and L, will be a straight
line with slope L
and L, will be a straight
line with slope L  and
intercept zero. A typical colorimetric analysis uses a series of
solutions, of known concentrations, to produce a Beer's Law calibration
plot, whose slope can be used to calculate
and
intercept zero. A typical colorimetric analysis uses a series of
solutions, of known concentrations, to produce a Beer's Law calibration
plot, whose slope can be used to calculate  . Then an unknown sample is
analyzed by measuring its absorbance. The
. Then an unknown sample is
analyzed by measuring its absorbance. The  (or slope) from the Beer's
Law plot is then used to relate the absorbance of the unknown to its
concentration in solution. Colorimetric analyses are normally carried out
at the wavelength of an absorption maximum since the absorption
coefficient is least sensitive to changes in wavelength near a
maximum.
(or slope) from the Beer's
Law plot is then used to relate the absorbance of the unknown to its
concentration in solution. Colorimetric analyses are normally carried out
at the wavelength of an absorption maximum since the absorption
coefficient is least sensitive to changes in wavelength near a
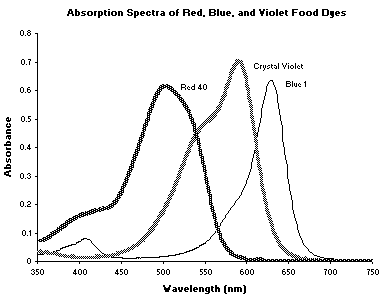
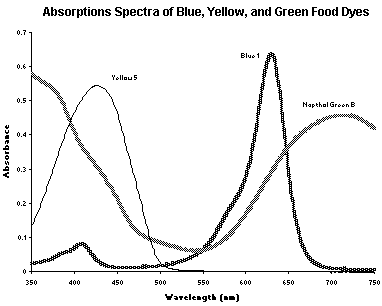
maximum.Your Koolaid sample may contain a single dye or a mixture of dyes. Absorption spectra and chemical structures of some common food dyes, which may be in your drink mix, are shown in the following pages. Stock solutions of these dyes with known concentration will be available in the lab for your use in the analysis of your Koolaid sample. If your drink solution contains more than one dye, the wavelengths for analysis must be chosen carefully. The wavelengths at which each dye is detected should show absorbance of only the dye being studied, not the other dye(s) in the mixture.
Paper Chromatography
A paper chromatography experiment will help you decide if your drink mix contains one dye or a mixture of dyes. Although your procedure is very simple, chromatography is a general and powerful technique for the separation of complex mixtures. In paper chromatography, a small amount of sample mixture is placed on a strip of paper and a solvent is used to separate its components. The strip of paper is placed with one end in the solvent. The liquid is then absorbed by the paper and travels up the strip, taking the sample with it. Some mixture components will move readily with the solvent up the paper and others will stick more stubbornly to the paper, not moving or moving more slowly. The degree to which a component sticks or moves depends on the intermolecular forces between the molecules of the substance and the paper or solvent. In this way, different substances can be separated and identified. Because Koolaid and the dye(s) it contains are brightly colored, you will be able to observe any dye separation on the paper visually.
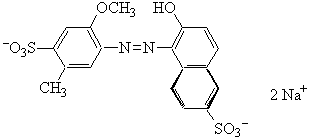
Allura Red or Red 4
MW = 496.43 g mol-1
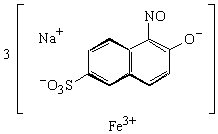
Napthol Green B or Acid Green
MW = 878.47 g mol-1
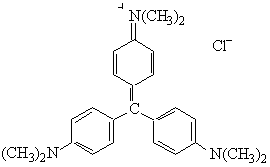
Crystal Violet or Basic Violet
MW = 407.99 g mol-1
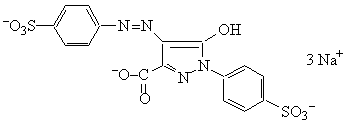
Tartrazine or Yellow
MW = 534.37 g mol-1
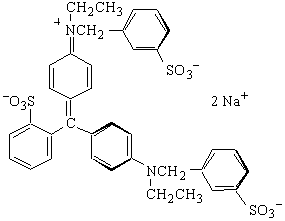
Erioglucine or Blue
MW = 792.86 g mol-1
Trustees of Dartmouth College, Copyright 1997–1999