Ingredients --
Starch and Modified Starch
Chemical Formula:
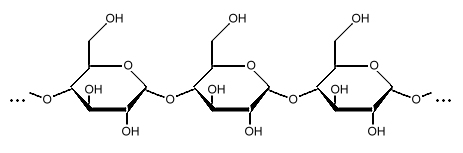
Amylose
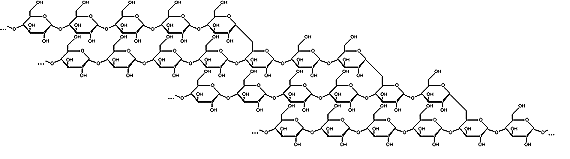
Amylopectin
Synonyms
Description
Starch is a polysaccharide, a chain of many glucose molecules. It is the main carbohydrate store in roots and seeds. There are two types of glucose chains in starch. One is a simple chain called amylose, and the other is a complex branched form called amylopectin. In the starch grains in a plant, amylopectin makes up the bulk of the material, between 50 to 80 percent by weight, made up of several million amylopectin molecules per starch grain. The rest is a much larger number of the smaller amylose chains, made up of 500 to 20,000 glucose units in each chain. Amylopectin molecules are made of several million glucose units.
Amylopectin Amylopectin forms branched structures with about 30 glucose units in a chain between branches. This makes the molecule somewhat striped in appearance, with the knotted branch points all in a row, and the smooth chains separating them. These molecules are so large that this striped appearance shows up under a light microscope, forming what appear to be "growth rings" in the starch grain.
Uses
Starch is a major source of calories in grains and tubers, and foods made from them. When starch is added to products as an ingredient, however, it is the functional properties of the starch that are usually important, not the calories. Starch is the main thickener in gravies, sauces, and puddings. It absorbs water, and becomes a gel when cooked. As the starch swells up with water, the amylose leaches out, and the amylopectin forms the gel. Some starches have higher amylopectin content, and make better gels than those containing lots of amylose. As a thickener (as opposed to a gel), it is the amylose that has the main function. The long water-soluble chains increase the viscosity, and that viscosity doesn't change much with temperature. Amylose chains tend to curl up into helices (spirals) with the hydrophobic parts inside. This allows them to trap oils and fats inside the helix, as well as aroma molecules. Because starches are so good at absorbing water and bulking up, they are important in the "mouth feel" of many food products, and are used as fat substitutes. Not all of the starch in a food ends up being digested. The starch that is not absorbed by the body is called "resistant starch", and is consider dietary fiber. It is also a source of nutrition for intestinal flora, which make important vitamins (and intestinal gas). Starches are added to processed meats (lunch meats, hot dogs, sausages, etc.) as a filler, binder, moisture retainer, and fat substitute. They are added to soups, sauces and gravies as thickeners. They are used in extruded cereals and snacks to hold the shape of the material.Modified starch
Starches can be modified in several ways to change their function as additives in products. They can be cross-linked, where the chains get stuck together into a mesh. They can be heated to break the long chains down into simpler molecules like dextrin, polydextrin, and malto-dextrin. These are simply short starches. Starches can have a hydrogen replaced by something else, such as a carboxymethyl group, making carboxymethyl starch.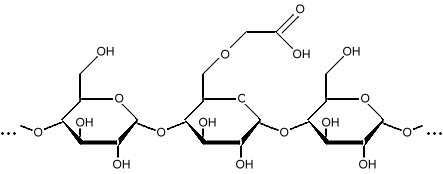
Carboxymethyl starch Adding the carboxymethyl group makes the starch less prone to damage by heat and bacteria. Carboxymethyl starch is used as an additive in oil drilling mud, and is used in the goo that makes ultrasound examinations messy. Carboxymethyl starch is also called a starch ether, or misnamed starch glycolate due to a historical misunderstanding. Carboxymethyl groups make the starch more hydrophilic (water loving), and aid in cross-linking. This makes carboxymethyl starch useful in aspirin and other tablets to make them disintegrate quickly. Longer carbon chains can also be added, such as carboxyethyl groups, or carboxypropyl groups. Adding bulky functional groups like carboxymethyl and carboxyethyl groups reduces the tendency of the starch to recrystallize. When the starch stays as a gel, a product is softer, and we say it is "fresh". When the starch regains its crystalline form, the product becomes firmer, and we say it is "stale". The technical term for this is starch retrogradation. Starches can be esterified by modifications with an acid. An ester is the result of reacting an alcohol with an acid. The starch loses a hydroxyl group, and the acid loses a hydrogen. These combine to form water as the other product of the reaction. Using acetic acid, starch acetates are formed, which are used as film-forming polymers for pharmaceutical products, and as the polymer in biodegradable packing foam "peanuts". Starch acetates have a lower tendency to create gels than unmodified starch. Acids can also break the long chains into shorter molecules, much like heat does, to form polydextrins, malto-dextrin, or dextrin. Enzymes are also used to do the same thing. Cross-linking occurs when a hydroxyl group (OH) on one chain bonds with a hydroxyl group on an adjacent chain. This toughens the starch, and helps it resist heat and acids. Cross-linking can be done by heating, or by reacting with compounds such as phosphates, or glycerol. Starches are also sometimes "pregelatinized" to make them easier to dissolve during product manufacture. Starches, especially modified starches, are also used as glues in cardboard manufacturing. Starches such as Gum Arabic and Gum Tragacanth are used as the glue for stamps and postal envelopes. Oxidized starch, usually oxidized with sodium hypochlorite, is whiter than unmodified starch, has increased clarity, and a lower viscosity.