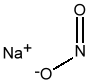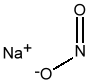Ingredients --
Sodium nitrite
Chemical Formula: NaNO2
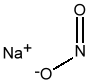
Synonyms
Nitrous acid sodium salt
Description
White or yellowish powder.
Uses
Sodium nitrite is used to fix the colors in preserved fish and
meats. It is also important (along with sodium chloride)
in controlling the bacterium
Clostridium botulinum, which causes botulism.
Lunch meats, hams, sausages, hot dogs and bacon are usually
preserved this way.
In medicines, it is a vasodilator, intestinal relaxant,
bronchodilator, and an antidote to cyanide poisoning,
and hydrogen sulfide poisoning.
Sodium nitrite is produced in the human body by the action
of saliva on
sodium nitrate,
and is important in controlling bacteria in the stomach,
to prevent gastroenteritis. The body produces more
sodium nitrite than is consumed in food.
Sodium nitrite can react with proteins in the stomach or
during cooking (especially in high heat, such as frying bacon)
to form carcinogenic
N-nitrosamines. To prevent this,
ascorbic acid
or
erythorbic acid
are now added to the cured meat.
During the curing process, some of the nitrites are converted
to nitric oxide. This combines with the myoglobin proteins
in the muscle of the meat to form the deep red nitric oxide
myoglobin, which turns bright pink during the smoking process.
This is why ham is pink.
Nitrites prevent foods from getting rancid. Besides the taste
issues,
nitrites prevent the formation of toxic maldonadehyde, which
is formed as foods get rancid.