Ingredients --
Ammonium Lauryl Sulfate
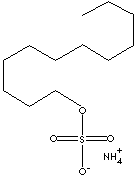
Chemical Formula: CH3(CH2)10CH2OSO3NH4
Synonyms
Dodecyl ammonium sulfate
Description
Light yellow, viscous liquid
Uses
Ammonium lauryl sulfate is an
anionic surfactant.
This means it lowers the surface tension of water, making
the water spread more easily. Surfactants are also called
wetting agents, and are said (somewhat paradoxically) to make
water "wetter".
Ammonium lauryl sulfate is added to products as a foaming
agent, and as a detergent.
Notice in the structural formula that one end of the molecule is
a long chain of carbon and hydrogen, while the other end is a
salt of sulfuric acid and ammonia. The long chain is hydrophobic,
and the salt is hydrophilic, making this a good
detergent and surfactant.
Ammonium Lauryl Sulfate is used in many shampoos, toothpastes, and
skin cleansers. It can be used in hard water.
When ammonium lauryl sulfate is reacted with ethylene oxide,
the result is the larger molecule ammonium laureth sulfate.
This larger molecule has the same detergent and surfactant
qualities, but is larger, so it does not penetrate the skin
and hair as easily. The term "laureth" is actually a contraction.
The full name is "ammonium lauryl ether sulfate".
Sodium Lauryl Sulfate is the same compound and has the same uses,
but the ammonium group has been replaced with a sodium atom.

