Ingredients --
Ammonium Lauryl Sulfate
Chemical Formula: CH3(CH2)10CH2OSO3NH4
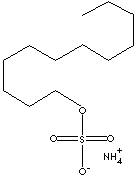
Synonyms
Dodecyl ammonium sulfateDescription
Light yellow, viscous liquidUses
Ammonium lauryl sulfate is an anionic surfactant. This means it lowers the surface tension of water, making the water spread more easily. Surfactants are also called wetting agents, and are said (somewhat paradoxically) to make water "wetter". Ammonium lauryl sulfate is added to products as a foaming agent, and as a detergent. Notice in the structural formula that one end of the molecule is a long chain of carbon and hydrogen, while the other end is a salt of sulfuric acid and ammonia. The long chain is hydrophobic, and the salt is hydrophilic, making this a good detergent and surfactant. Ammonium Lauryl Sulfate is used in many shampoos, toothpastes, and skin cleansers. It can be used in hard water. When ammonium lauryl sulfate is reacted with ethylene oxide, the result is the larger molecule ammonium laureth sulfate. This larger molecule has the same detergent and surfactant qualities, but is larger, so it does not penetrate the skin and hair as easily. The term "laureth" is actually a contraction. The full name is "ammonium lauryl ether sulfate". Sodium Lauryl Sulfate is the same compound and has the same uses, but the ammonium group has been replaced with a sodium atom.ammonium lauryl sulfate: InChI=1/C12H26O4S.H3N/c1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15;/h2-12H2,1H3,(H,13,14,15);1H3/fC12H25O4S.H4N/h;1H/q-1;+1
By Simon Quellen Field