Octanitrocubane - The most powerful explosive
Carbons like to form bonds with angles of 109 degrees, like the bonds in diamonds. It takes energy to force the bonds to form more acute angles. The highest strain energy of any organic compound available in multi-gram amounts is found in cubane, a molecule with eight carbons in a cube shape, where all of the bonds are 90 degrees.
In the early 1980's it was pointed out that cubane's very high density and high heat of formation would make it an especially good explosive, especially if each carbon could have a nitro group attached. The resulting molecule would decompose to eight molecules of carbon dioxide, and four molecules of nitrogen, and release a lot of heat in the process. A cubane with a nitro group on each carbon is called octanitrocubane.
Several factors are important in making a good explosive. The decomposition must be energetic. In cubane derivitives, the strain energy ensures a very energetic decomposition. The number of molecules that result should be large. One molecule of octanitrocubane becomes 12 molecules, a very good ratio for an explosive. The molecular weight of the resulting gases should be high. Carbon dioxide's molecular weight is 44, and nitorgen's is 28, compared to water at only 18. Explosives that contain hydrogen will have lighter resulting molecules than those that don't, and octanitrocubane has no hydrogen.
Lastly, the density of the substance should be as high as possible. A denser explosive has a higher detonation velocity (more power), and a higher maximum detonation pressure. Octanitrocubane is the densest explosive yet.
Because it has no hydrogen, it produces no water vapor. When used as a propellant in rockets, this would mean that it would leave no vapor trail, and it would be harder to detect and track.
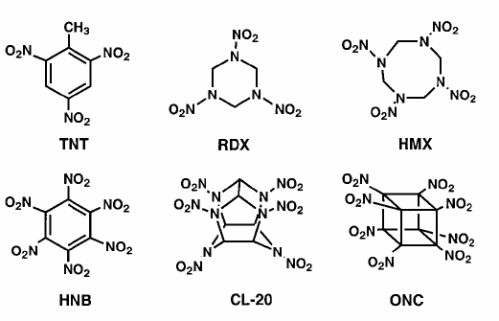
Some common explosives and their properties:
| Name | Density | DetonationVelocity | DetonationPressure |
| TNT | 1.6 | 7.0 | 190 |
| RDX | 1.8 | 8.8 | 338 |
| HMX | 1.9 | 9.1 | 390 |
| HNB | 2.0 | 9.4 | 406 |
| CL-20 | 2.0 | 9.4 | 420 |
| ONC | 2.1 | 10.1 | 500 |
Octanitrocubane is up to 30% more powerful than the most powerful common military explosive, HMX. What is also important, it is very stable. It does not degrade until it sublimes at 200 degrees Celsius (392 degrees Fahrenheit). You can hit it with a hammer and it will not explode.
Perhaps fortunately, it is very difficult to make. Cubane itself is not easy, but then a 17 step process is needed to get to tetranitrocubane, and by the time we get to hexanitrocubane we are at 33 steps, then heptanitrocubane brings us to 37 steps, and finally octanitrocubane at 40 steps. Many of those steps in the reaction are quite difficult all by themselves.
Other cubane derivitives have useful properties too. They are being explored for their pharmaceutical uses, and for their unique qualities when polymerized into plastics.

3 Comments:
Simon, where the heck do you get all this cool, geeky information?
Your blog is orders of magnitude more interesting than /.
Thanks!
I read a LOT of scientific technical periodicals, such as Science, Nature, Physics Today,
American Journal of Physics, The Physics Teacher, etc.
Very nice blog. I read you from Valencia, Spain. A lot of thanks.
Jose Luis
Post a Comment
Links to this post:
Create a Link
<< Home